Table of Contents
🔎 30-Second Summary
Bismuth isn’t just a colorful oddity—it’s a rising star in modern industry. This article uncovers how bismuth is quietly replacing toxic metals in medicines, fire safety devices, and cosmetics, while also shaping the future of electronics.
You’ll explore its role in low-melting alloys, shotgun ammunition, and even next-gen semiconductors like bismuthene and topological insulators.
With applications spanning from eco-friendly solders to quantum transistors, bismuth’s strategic importance is growing fast. Whether you’re a science enthusiast or a policy follower, this deep dive reveals why bismuth deserves far more attention than it gets.
Most people don’t expect heavy metals to be used in medicines, cosmetics, or fire sprinklers, but bismuth does all three. It’s dense but gentle, and that rare combination makes it useful in surprising places, from pink stomach tablets to eco-friendly plumbing and semiconductor chips.
Its shiny, colourful crystals were once confused with lead or tin. Even alchemists got it wrong for centuries. The name “bismuth” comes from the German Wismut, with older roots in Latin as bisemutum. Ancient metalworkers might have added it to bronze without knowing it.
Today, bismuth stands out for being safe, versatile, and a good replacement for toxic metals. To see why, we need to start at the elemental level.
Bismuth’s Availability and Production
Though rarely mined as a primary metal, bismuth plays a critical role in the global supply chain, with its availability heavily tied to its extraction as a byproduct of other base metal ores.
Occurrence in nature
Bismuth typically occurs in the Earth’s crust at concentrations of about 0.008 ppm. It’s most often found in the form of bismuthinite (Bi₂S₃) and bismite (Bi₂O₃), alongside lead, copper, tin, and tungsten deposits.
These geological ties mean bismuth is generally recovered as a by-product during the smelting of other metals.
Pure native bismuth is rare, usually forming in hydrothermal quartz veins, and despite its low crustal abundance (~0.00002%), it tends to concentrate in specific polymetallic deposits.
Global production and reserves
According to the USGS, China dominates global bismuth production, accounting for more than 70% of the world’s output. Other key contributors include Mexico, Bolivia, and Peru. While global reserves are modest compared to more common metals, current demand remains manageable due to low-volume applications.
Byproduct recovery and processing methods
Most commercial bismuth comes from refining lead concentrates, especially in regions where environmental regulations restrict the use of lead and boost demand for substitutes.
Hydrometallurgical methods are increasingly favoured, using leaching, solvent extraction, and precipitation to isolate high-purity bismuth from complex ores. As demand grows in pharmaceuticals, electronics, and green tech, securing stable by-product flows is becoming a strategic priority for many countries.
What is Bismuth and Why It’s Unique
Bismuth (atomic number 83) is a post-transition metal in Group 15 of the periodic table, but its behaviour is anything but typical. It is dense but safe, striking in appearance but stable under pressure.
Unlike toxic neighbours like lead or mercury, bismuth is chemically stable, environmentally safer, and forms some of the most visually striking crystals in the mineral world.
When bismuth cools from a melt, it forms intricate hopper crystals with geometric edges and vibrant colours. These colours arise from a thin oxide layer that creates iridescence—a rainbow-like effect caused by light interference.
💡 Did You Know?
Bismuth crystals expand when they solidify—unlike most metals—forming beautiful hopper shapes that look like fractals!
That mix of function and gentleness makes it more than just another post-transition metal. It is often recovered as a by-product during the refining of lead, copper, tin, or tungsten ores. Yet, despite its secondary recovery status, its industrial significance has been rising, thanks to its ability to replace more harmful substances in consumer and industrial applications.
Let us unpack what makes bismuth so unique, not just in the periodic table, but across industries.

Physical and Chemical Properties
Bismuth is a brittle, silver-white metal with a pinkish tinge. It crystallises in a rhombohedral structure and melts at around 271°C, making it useful for low-temperature alloys and safety fuses.
These are often classified as fusible alloys—engineered metal blends that melt at predefined temperatures to trigger thermal cut-offs in fire sprinklers, circuit breakers, and pressure valves.
Bismuth also expands slightly when it solidifies, a rare trait among metals, making it valuable in casting and precision moulds, and with a high density (9.78 g/cm³) but low toxicity and good stability, making it safer than neighbouring heavy metals like lead. Its low thermal conductivity and high electrical resistance enable its use in heat control and electrical components.
Stability and compound formation
Bismuth is highly chemically stable, resisting oxidation and remaining inert under most conditions, especially when compared to more reactive metals like aluminium or magnesium. It forms a wide variety of stable compounds in the +3 oxidation state, including oxides, halides, and nitrates.
Many of these adopt layered or polymeric structures, enhancing their stability. This chemical’s predictability and low reactivity support its use in pharmaceuticals, pigments, and environmental materials.
Diamagnetism and low toxicity
Bismuth is the most diamagnetic of all naturally occurring elements, meaning it repels magnetic fields more strongly than any other metal found in nature. This unusual trait, paired with its low thermal conductivity, makes it useful in superconducting systems and magnetic shielding technologies.
Unlike other heavy metals such as lead, bismuth does not bioaccumulate in the body and is considered extremely low in toxicity. Many of its compounds are approved for medical and cosmetic use, making it a rare example of a heavy metal that is both physically and biologically safe for everyday applications.
Bismuth(III) vs Bismuth(V) compounds
Bismuth predominantly forms compounds in the +3 oxidation state, such as bismuth oxide (Bi₂O₃) and bismuth subnitrate, which are used in catalysts, pigments, and medicines. The +3 state is favoured due to its greater energetic stability and the inert pair effect, which limits the involvement of 6s electrons in bonding.
While +5 compounds like bismuth pentafluoride (BiF₅) do exist and are typically limited to niche roles in fluorination chemistry or as oxidising agents in advanced material synthesis, they are rare and less stable.
Comparisons to other heavy metals in medicine
Bismuth stands apart from toxic heavy metals like lead, cadmium, and mercury, as it does not pose health risks at typical exposure levels. Its compounds, such as bismuth subsalicylate and subnitrate, are widely used in antacids, ulcer medications, and dermatological products, with well-established safety records.
This safety profile, reinforced by decades of clinical use, has made bismuth a preferred substitute in pharmaceuticals and consumer goods as other heavy metals are increasingly phased out.
These unusual physical and chemical traits are precisely what make bismuth so versatile, from soothing stomachs to enabling semiconductors.
Bismuth in Medicine: A Gentle Heavy Metal
From pink stomach relief tablets to antimicrobial treatments for ulcers, bismuth plays a quiet yet vital role in modern medicine. Its low toxicity, broad-spectrum antimicrobial properties, and continued effectiveness where antibiotics or acid-neutralisers may struggle have made it a cornerstone in select pharmaceutical formulations, especially in gastrointestinal and topical applications.
Bismuth subsalicylate uses in digestive health.
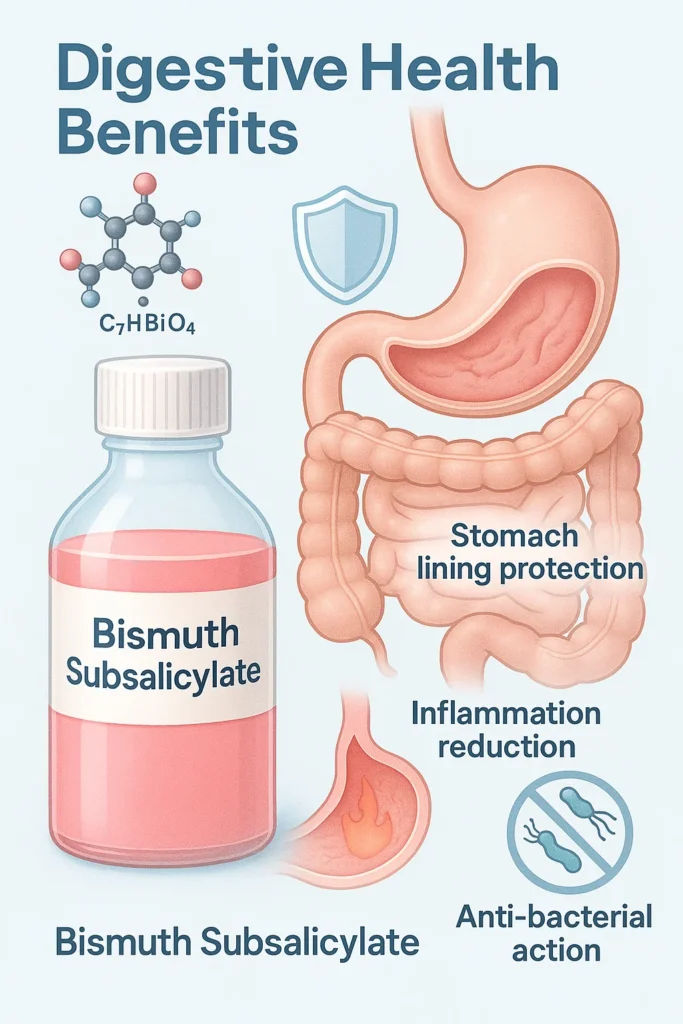
Bismuth subsalicylate, a combination of bismuth and salicylic acid, is the active ingredient in over-the-counter medicines like Pepto-Bismol and is widely used to treat diarrhoea, heartburn, and nausea. It works by coating the stomach lining, reducing inflammation, and slowing the activity of certain harmful bacteria.
This dual action makes it especially effective for managing mild gastrointestinal discomfort, including traveller’s diarrhoea. According to PubChem, it also shows efficacy against Helicobacter pylori, a bacterium linked to peptic ulcers.
Its unique value lies in combining antimicrobial and anti-inflammatory effects, a rare trait among non-antibiotic elements. A typical Pepto-Bismol tablet contains approximately 262 mg of bismuth subsalicylate, delivering a low but effective dose of bismuth ions for short-term gastrointestinal relief.
Bismuth subnitrate and subcarbonate applications
In contrast, bismuth subnitrate and bismuth subcarbonate are more common in prescription formulations. These salts serve as protective agents, especially in combination therapies. They help form a barrier on the stomach or duodenum, protecting the mucosa from acid and aiding ulcer recovery.
These compounds are often added to topical creams, wound powders, and ointments due to their soothing effects on irritated tissue. They were also historically used in treating syphilis before antibiotics became widespread.
Bismuth tablet uses and safety profile.
Bismuth medications are available in both solid and liquid forms. Chewable tablets and capsules are used for self-care in cases of mild digestive discomfort, while injectable or compound formulations may be administered under clinical supervision.
Unlike other heavy metals, bismuth compounds are minimally absorbed in the gastrointestinal tract, and most of the element is excreted without entering the bloodstream.
This low absorption limits systemic toxicity, making bismuth tablets safe for short-term use, even in vulnerable populations. However, overuse or prolonged exposure can lead to rare neurological effects, making regulatory oversight essential.
Low Toxicity in Pharmaceuticals
Bismuth is recognised by the European Medicines Agency and other global regulators for its favourable safety profile, particularly in short-term medical use. Unlike lead or mercury, it does not accumulate in the body, which significantly lowers the risk of long-term toxicity.
Side effects are rare and generally mild, the most common being temporary darkening of the tongue or stools, both harmless and fully reversible.
Other pharmaceutical uses (eye drops, internal deodorants)
Beyond gastrointestinal care, bismuth is also used in ophthalmic solutions, internal deodorants, and antiseptic wound treatments. These applications benefit from its mild bacteriostatic effect and its ability to bind foul-smelling sulphur compounds in the gut.
In some cases, bismuth oxide is also added for radiopacity in X-ray-visible fillers. These niche uses rely on its antibacterial action and chemical stability, critical traits for products targeting sensitive areas.
With a strong safety record and proven clinical utility, bismuth remains an underrated but valuable tool in both frontline medicine and specialised pharmaceutical formulations.
This same versatility explains its surprising presence in cosmetics and pigments, the focus of the next section.
Bismuth in Cosmetics and Pigments
Beyond medicine, bismuth is a staple in cosmetics and pigments, valued for its shimmer, skin compatibility, and colour stability, ideal for everything from highlighters to eyeshadows. Unlike other metallic additives, its low toxicity and dermatological safety help it remain relevant even under strict regulatory frameworks.
Bismuth Oxychloride in Makeup
The standout compound in this space is bismuth oxychloride (BiOCl). Known for its pearlescent sheen, it is widely used in face powders, blushes, and mineral foundations. The layered crystal structure reflects light in a way that creates a natural glow, often marketed as a “soft-focus” effect.
Its unique optical properties have made it a favourite in premium makeup, particularly in formulations marketed for a radiant or soft-glow finish.
Bismuth Uses in Cosmetics and Pigments
Beyond Bismuth oxychloride (BiOCl), bismuth-based pigments appear in nail polishes, lipsticks, and creamy makeup products. When combined with sulphur, titanium dioxide, or iron oxides, they yield colours from pearly white to pink and bronze.
Their chemical stability ensures long shelf-life without discolouration or reactivity with other ingredients, a critical trait for commercial beauty products. In some specialised products, bismuth compounds are used as both colouring agents and structural enhancers, improving product texture and adhesion.
Safety and Skin Compatibility
Unlike older cosmetic compounds like lead or arsenic, bismuth oxychloride is considered safe for topical use. Its flat crystalline structure helps prevent pore blockage and reduces allergic reactions.
Unlike older cosmetic compounds like lead or arsenic, bismuth oxychloride is considered safe for topical use. Its flat crystalline structure helps prevent pore blockage and reduces allergic reactions. Bismuth oxychloride is often tolerated better than substances like talc or mica, which can irritate delicate skin.
Some users may experience mild itching or heat sensitivity with prolonged use. However, regulatory authorities such as the FDA and the EU’s Scientific Committee on Consumer Safety continue to classify it as safe for topical use.
Pearlescent Effects in Nail Polish and Eyeshadow
Bismuth’s flake structure creates a multidimensional shimmer in nail polishes and eyeshadows, mimicking pearl or opal finishes. This allows brands to achieve high-luxury effects without using animal-derived or plastic-based additives, especially in premium product lines.
The result is a luminous, long-lasting finish that resists smudging and fading. Unlike synthetic mica or plastic-based glitters, bismuth-based shimmer offers a more natural, less abrasive alternative.
Historical Use in Skin Whitening
In the 19th century, bismuth compounds such as bismuth subnitrate were used in skin-whitening powders across Europe and Japan, an early example of its role in appearance-related formulations.
Its transition from traditional cosmetics to modern clean-beauty brands shows how legacy ingredients are being reimagined with safety and performance in mind. Today, it remains a staple in mineral-based and cruelty-free beauty lines.
While bismuth brings a soft glow to cosmetics, it plays a much tougher role in the next sector, metallurgy. Its low melting point and lead-replacement potential make it invaluable in fire safety, machining, and metal casting.
Bismuth in Alloys and Metallurgy
Bismuth’s metallurgical versatility lies in its ability to form low-melting, lead-free, and environmentally safer alloys across multiple industries, from electronics to safety engineering.
Lead-Free Alloys and Soldering
Bismuth is commonly used in solder alloys with tin, indium, cadmium, and occasionally small amounts of lead. These are essential in circuit boards, medical devices, and high-voltage systems due to their RoHS compliance and suitability for heat-sensitive components. Notably, bismuth–indium alloys like “Solder 174” (57% Bi, 26% In, 17% Sn) melt at just 79 °C.
Low-Melting Fusible Alloys
Alloys such as Wood’s metal (Bi, Pb, Sn, Cd) and Rose’s metal (Bi, Pb, Sn) rely on bismuth for their precise melting behaviour. These fusible alloys (low-melting-point mixtures that flow under heat, used in fire safety and moulding) are indispensable in fire suppression systems, sprinkler links, mould making, and thermal fuses, thanks to their ability to transition at controlled temperatures.
Lead–Bismuth Thermal Alloys
Lead–bismuth alloys containing 30–75% bismuth offer a nearly zero volume change on melting, making them ideal for sealed systems, nuclear coolant applications, and speciality casting. Their phase stability is crucial in environments that demand dimensional accuracy and repeatability under heat.
Corrosion-Resistant and Free-Machining Alloys
In copper alloys like bismuth bronze, bismuth adds corrosion resistance and machinability, reducing tool wear and enabling precision in valves, bearings, and fittings. In free-machining steel, bismuth replaces lead to enhance chip formation while maintaining recyclability and workplace safety.
Free-Machining Applications in Consumer Hardware
In brass, steel, and aluminium alloys, bismuth improves chip formation and surface finish, key traits in “free-machining” materials. These alloys are widely used in manufacturing household hardware such as door handles, plumbing fittings, and electrical terminals, where smooth production and tight tolerances are critical.
Adding bismuth allows manufacturers to cut cleaner, reduce tool wear, and produce less scrap, supporting both cost efficiency and environmental goals.
Bismuth’s value often hides in plain sight In domestic systems, it reinforces fire safety, boosts the performance of home fixtures, and makes everyday components easier to machine and assemble.
Whether in thermal links or tap fittings, its role is subtle but increasingly essential, especially as industries shift away from hazardous metals. By combining performance with safety, bismuth proves that even a small additive can have a large-scale impact, from factories to homes and everywhere in between.
Lesser-Known Industrial and Artistic Uses
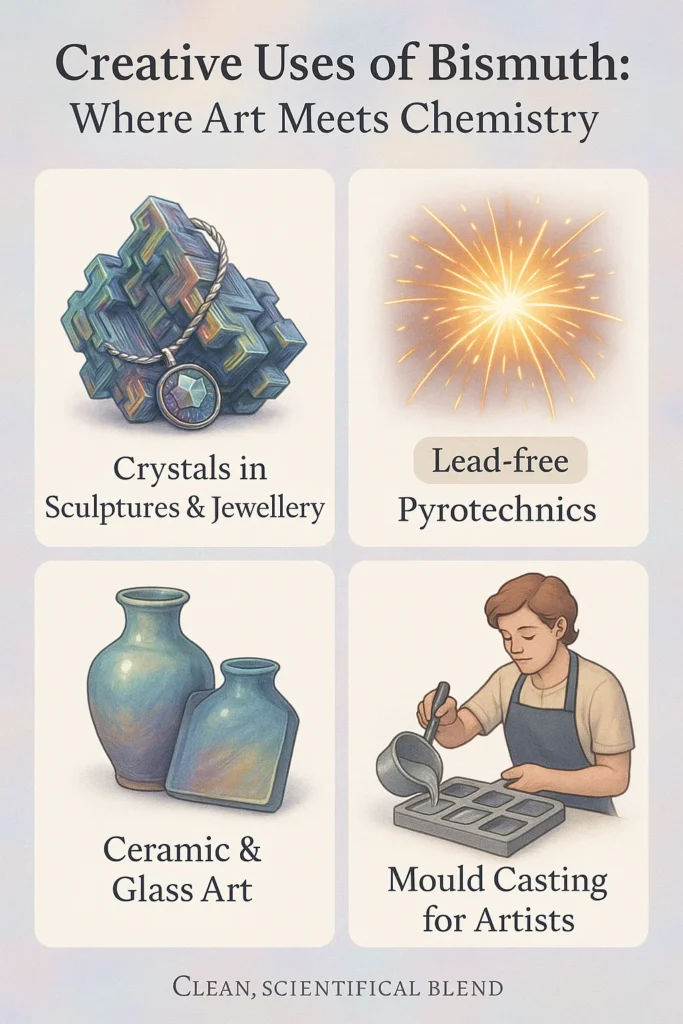
Bismuth’s appeal goes beyond metallurgy and electronics, entering the realms of art, tradition, and craftsmanship. Its low toxicity and crystalline beauty make it a favourite in settings where aesthetics meet functionality.
Decorative Crystals and Sculptural Art
Bismuth’s tendency to form iridescent hopper crystals has inspired its use in artisanal sculptures, jewellery, and educational mineral displays. These rainbow-toned formations occur naturally during controlled solidification and are often sold as collector’s pieces or novelty décor. Though not widely commercialised, this niche market highlights the metal’s visual appeal and safe handling properties.
Pyrotechnics and Thermal Effects
In small-scale pyrotechnics, bismuth compounds occasionally substitute for lead to create colourful spark effects and temperature-triggered displays. While not a major segment, their inclusion helps reduce toxic metal content in fireworks and thermally reactive materials.
Pigment Craft and Glass Art Crossover
Bismuth-based glazes and enamels have long been used in heat-resistant pottery and art glass. Artisans used compounds like bismuth subnitrate and trioxide to give ceramic works durability and lustre. These applications continue in small workshops and experimental studios today.
Bismuth in Ammunition and Hunting Shot
One of the earliest public-facing examples of bismuth’s environmental value is its use in ammunition. As lead shot was banned in wetland hunting due to its toxic effect on birds and aquatic life, bismuth–tin shot became a legal and safer replacement.
Unlike steel shot, which can damage gun barrels and lacks density, bismuth offers similar ballistic properties to lead while remaining nontoxic. Its soft impact, density, and corrosion resistance make it ideal for vintage firearms and environmentally sensitive areas alike, blending tradition with ecological responsibility.
Bismuth in Medical Imaging and Radiation Shielding
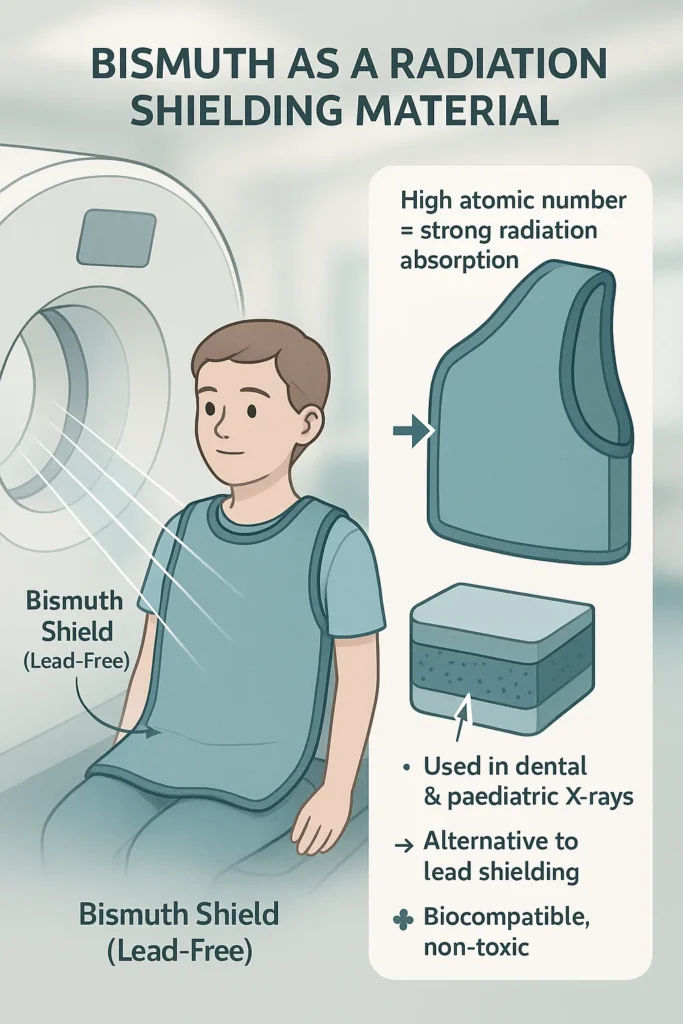
Bismuth also finds critical use in radiation shielding, particularly in medical imaging and X-ray diagnostics. While lead remains common, bismuth-based composites are now used in protective vests and shielding curtains to reduce patient exposure.
These materials block ionising radiation effectively while being lighter, safer to handle, and less hazardous during disposal. Some hospitals have even shifted to bismuth-based creams to shield sensitive areas during CT scans, highlighting how medical-grade bismuth is improving both safety and patient comfort.
Whether it’s shielding patients from radiation or replacing toxic metals in consumer goods, bismuth shows that heavy metals don’t have to come with heavy consequences. Its eco-friendly role sets the stage for future innovations, where safety and sustainability go hand in hand.
Smart Materials and Shape Memory Alloys
Recent research has also begun to explore bismuth’s suitability for shape memory alloys (SMAs), smart materials that can recover their original shape when heated. Although still in early stages, these developments point to bismuth’s potential in next-generation actuators, sensors, and thermal control systems, particularly where non-toxic alternatives are needed.
Moulding for Artistic Castings
Low-melting bismuth alloys, originally developed for industrial casting, have also been adopted in creative spaces. These alloys allow artists and craftspeople to cast intricate shapes without high-temperature kilns or specialised equipment, enabling DIY mould-making for sculptures, medallions, or prototypes.
From galleries to glass kilns, bismuth’s versatility gives it a quiet yet distinct role in niche creative industries, merging safety, science, and self-expression.
Electronics and Semiconductor Innovations
Bismuth’s unique properties are reshaping how modern electronics are cooled, assembled, and optimised. From thermoelectric chips to next-gen transistors, this heavy yet gentle metal is quietly emerging as a materials science enabler.
Bismuth Telluride and Thermoelectric Cooling
Bismuth telluride (Bi₂Te₃) and bismuth selenide (Bi₂Se₃) are widely used thermoelectric materials (which convert heat differences directly into electrical energy) that convert heat differences into electricityusing the Seebeck effect. This enables energy harvesting in microprocessors, wearables, and automotive systems.
What makes these compounds stand out is their rare ability to conduct electricity efficiently while resisting heat transfer, a key trait for passive cooling and thermal energy recovery. Bi₂Te₃, in particular, is favoured for fanless cooling modules in defence electronics, high-end cameras, and silent computing setups.
Beyond personal devices, bismuth-based thermoelectric systems also power satellites, industrial monitors, and remote sensors, in situations where mechanical cooling is impractical. The result is solid-state cooling that’s quiet, durable, and maintenance-light.
These systems support broader energy efficiency goals, cementing bismuth-based thermoelectrics as a cornerstone of green electronics design.
Bismuth in Transistors and Semiconductors
At the cutting edge of semiconductor research, bismuth is drawing interest for its strong spin–orbit coupling, a quantum property useful in low-power electronics. In topological insulators, bismuth-based compounds like Bi₂Se₃ and Bi₂Te₃ allow electricity to flow only on the surface, with the interior acting as an insulator.
This unusual behaviour has implications for spintronics, where electron spin (not just charge) is used for data processing.
Another breakthrough is bismuthene, a two-dimensional monolayer similar to graphene, with potential for high-speed transistors and ultra-efficient logic gates.
Engineers are also testing bismuth-based interfaces to reduce contact resistance in advanced chips, a major concern as devices shrink to the nanoscale. From spin-based memory to power-efficient processors, bismuth is fuelling semiconductor innovation beyond traditional silicon.
Low-Melting Solders for Circuit Boards
Bismuth–tin (Bi–Sn) and bismuth–silver (Bi–Ag) solders are now widely used in place of lead-based ones for assembling printed circuit boards (PCBs). Their low melting points help reduce thermal stress, especially when working with heat-sensitive components like sensors, LEDs, and flexible electronics.
These solders also align with environmental regulations. As RoHS-compliant materials, they support green manufacturing while improving workplace safety and enabling recyclable electronics.
By combining safety, thermal control, and electrical reliability, bismuth-based solders are becoming standard in both consumer devices and industrial-grade systems.
India’s Role in Bismuth-Based Semiconductor Research and Innovation
India may not be a major producer of bismuth, but its relevance is growing in policy and research, particularly for semiconductors, defence systems, and thermoelectric technologies. As initiatives like Make in India and Semicon India gain traction, niche materials like bismuth are finding their way into strategic conversations.
Institutions such as IIT Madras and IISc Bangalore have initiated lab-scale research on bismuth-based thermoelectrics and spintronic materials, aiming to diversify beyond silicon-based electronics.
On the application front, agencies like DRDO and ISRO see potential in bismuth compounds for vibration-free cooling in satellite systems, remote sensors, and aerospace payloads, where durability and maintenance-free operation are critical.
Yet, India lacks a domestic supply chain for refined bismuth. Most of the demand is met via imports, whether as raw material or embedded within imported components. If India wants to lead in post-CMOS and energy-efficient electronics, securing access to speciality metals like bismuth will be crucial.
Environmental and Health Considerations
Bismuth’s growing popularity in modern materials science isn’t just about performance. It’s about responsibility. From reducing toxic exposure in consumer products to enabling greener manufacturing processes, this heavy metal plays a surprisingly gentle role in our environment and health landscape.
Why Bismuth Is Safer Than Lead and Mercury
Bismuth is often praised as the “green” alternative to lead and mercury, and for good reason. Unlike its toxic cousins, bismuth is chemically stable, biologically inert in small doses, and does not bioaccumulate. That means it doesn’t persist in the body or ecosystem the way lead and mercury do.
This safety profile makes it ideal for use in products like cosmetics, plumbing solder, and ammunition, all of which were traditionally dominated by lead-based materials. Its minimal reactivity and poor solubility also make accidental exposure far less dangerous, particularly in consumer or medical settings.
Compared to heavy metals known for neurotoxicity and organ damage, bismuth’s low toxicity is well documented in clinical and environmental studies.
Bismuth’s Role in Sustainable Materials and RoHS Compliance Standards
Bismuth isn’t just replacing lead; it’s reshaping how materials are selected for environmental compliance. In electronics, bismuth–tin and bismuth–silver solders are widely adopted as RoHS-compliant alternatives that maintain performance without toxic residues.
These low-melting solders reduce energy consumption during manufacturing and minimise the release of fumes or slag. In other industries, bismuth is added to alloys and coatings to improve environmental stability, lower waste output, and simplify disposal, particularly in applications like plumbing fixtures, safety devices, and household electronics.
Its alignment with green manufacturing goals has made bismuth a staple in sustainable material design, especially in sectors where safety and environmental impact are tightly regulated.
Global regulatory frameworks partly drive Bismuth’s growing popularity in metallurgical formulations. Under Europe’s RoHS (Restriction of Hazardous Substances) and REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) directives, bismuth is increasingly favoured as a substitute for lead, cadmium, and arsenic, especially in solders, plumbing components, and brass alloys. Its alignment with such compliance standards strengthens its role in sustainable materials innovation.
While generally considered safe, excessive ingestion of bismuth compounds over time may lead to side effects like bismuth encephalopathy—though such cases are rare and usually linked to overuse of medicinal formulations.
Future of Bismuth: Innovations, Markets, and Possibilities
As environmental pressures and technology demands evolve, bismuth is shedding its role as a quiet substitute and stepping into the spotlight. Future applications are no longer limited to “lead-free” alternatives; they are creating entirely new markets.
Bismuth in Advanced Materials and Catalysis
Beyond electronics and health care, bismuth is being explored for next-gen materials in catalysis and surface chemistry. Recent studies highlight its potential in CO₂ conversion, water splitting, and organic transformations, where bismuth-based catalysts offer selectivity and low toxicity compared to traditional heavy metals like palladium or platinum.
Researchers are also experimenting with bismuth oxides and nanosheets in photocatalysis and environmental remediation, aiming to harness solar energy or degrade pollutants sustainably. This points to a future where bismuth could support green chemistry at scale.
High-Purity Bismuth for Quantum and Spintronics
In high-tech labs, ultra-pure bismuth is gaining attention for its unique quantum properties. Thanks to its long electron coherence length and strong spin–orbit interaction, it’s being investigated for use in spintronics, a branch of electronics that leverages electron spin instead of charge.
This could lead to new types of low-energy data storage and logic devices that outperform silicon in both speed and efficiency. Bismuth’s role in topological insulators and Weyl semimetals is also opening doors in quantum computing research.
Bismuth Nanomaterials and Biomedical Frontiers
At the nanoscale, bismuth is proving to be more than just safe. Bismuth nanoparticles are being explored for targeted drug delivery, contrast agents in imaging, and even cancer therapy via photothermal methods.
Because bismuth is dense, stable, and biocompatible, it offers multiple pathways for innovation in non-invasive diagnostics and treatment. Its use in multifunctional nanomaterials may revolutionise how certain medical conditions are detected and treated.
Market Growth and Strategic Supply Chains
As demand for sustainable technologies rises, so does interest in securing a reliable bismuth supply. Countries with ageing lead smelters may struggle to keep pace, as bismuth is often produced as a byproduct of lead refining. This could create pricing volatility or supply constraints, especially if usage expands across high-value industries.
In response, several nations are exploring direct bismuth mining, improved refining techniques, and recycling strategies to reduce dependence on lead-linked sources. This shift from a secondary to a primary resource mindset may define bismuth’s future market trajectory.
As the global demand for bismuth rises across sectors, from medicine to green technology, questions around domestic access, policy support, and long-term sourcing become increasingly relevant, especially for emerging economies like India.
India’s Strategic Role and Global Position
While bismuth may not dominate headlines like lithium or rare earths, its role in strategic sectors is quietly growing. For India, the conversation around bismuth is still emerging, but the signals are worth watching.
Does India Produce or Import Bismuth?
India does not currently produce bismuth at scale. The mineral is not mined domestically in significant quantities and is typically obtained as a byproduct of lead or tungsten refining abroad. As of now, India relies entirely on imports for its bismuth needs, primarily sourcing from China and Belgium, the world’s largest producers and exporters.
This dependence creates vulnerabilities, especially as global demand for bismuth in electronics, green manufacturing, and medical devices rises.
Is Bismuth on India’s Critical Minerals List?
While bismuth may not grab headlines like lithium or rare earths, it has officially entered the spotlight in India’s mineral strategy. As of July 2023, bismuth was included in the Indian government’s list of 30 critical minerals, a clear sign that its value is no longer overlooked.
Bismuth’s recognition as a critical mineral marks a shift in national priorities. The government’s updated list reflects growing demand across key sectors such as green manufacturing, defence electronics, medical diagnostics, and RoHS-compliant industrial processes. Its inclusion not only acknowledges global trends but also anticipates India’s future industrial needs.
One of bismuth’s most strategic advantages lies in its role as a non-toxic substitute for lead in solders, pigments, ceramics, and radiation shielding. As India aligns with global environmental directives like RoHS and REACH, bismuth’s safer chemical profile gives it growing relevance in electronics, healthcare, and sustainable industrial manufacturing.
This move could drive new investments in exploration, recycling, and refining — areas where India currently lacks a mature infrastructure for bismuth handling. It also opens the door for public–private partnerships to reduce import dependency.
Role of KABIL, FDI, and International Partnerships
While KABIL (Khanij Bidesh India Ltd.) has so far focused on battery minerals like lithium, cobalt, and nickel, its framework could potentially expand to include bismuth and other emerging materials as industrial demand diversifies.
India has also opened its mining sector to 100% FDI in exploration, which could encourage private players to study bismuth-bearing ores within polymetallic zones, especially in regions rich in lead, copper, or tin.
Strategic collaborations with countries like Bolivia, Argentina, and Australia, known for their mineral diplomacy with India, could open new avenues for sourcing bismuth in cleaner, traceable supply chains.
Domestic Potential and Policy Gaps
Bismuth traces have been noted in select Indian deposits, particularly in lead–zinc–copper belts in Rajasthan and Odisha. However, there are currently no commercial projects extracting bismuth in these zones, partly due to a lack of targeted exploration.
The absence of a recycling strategy for bismuth compounds, especially from spent electronics and solder, is another missed opportunity. As India pushes toward circular economy models, targeted recovery of trace critical metals like bismuth could support both environmental and industrial goals.
India’s bismuth story is still unwritten, but with the right policy lens, it could become a quiet enabler in the country’s push for green tech, precision medicine, and electronic self-reliance.
Conclusion: A Heavy Metal with a Light Touch
Bismuth defies expectations. For a metal once mistaken for lead, it has carved out a role in everything from life-saving medicine to chip-scale cooling. Its unique traits, non-toxicity, visual brilliance, and thermal-electrical nuance make it an unusual but essential element in today’s material innovation landscape.
Yet, bismuth’s future hinges on recognition. It’s absent from many critical mineral lists, underutilised in India’s policy frameworks, and often overlooked in clean-tech discussions. As demand grows for safer, recyclable, and multifunctional materials, bismuth offers an elegant solution hiding in plain sight.
India now faces a pivotal question: will it invest in refining, recovery, and research for this unsung metalloid, or continue relying on global supply chains to meet emerging demand?
These hidden bismuth uses, ranging from medicinal compounds to eco-friendly alloys, show why this often-overlooked metal is gaining strategic attention worldwide.
💬 We’d love to hear from you!
Have you come across bismuth in surprising places? Or do you see it playing a bigger role in India’s green tech story? Drop your thoughts below and join the conversation.
What are some common uses of bismuth in everyday life?
Bismuth is used in various everyday products, including digestive medicines like Pepto-Bismol, cosmetics for pearlescent effects, fishing weights as a lead alternative,and in fire detection systems. It’s also found in plumbing fixtures and electronics as a safer replacement for lead.
Is bismuth safe for human consumption?
Yes, bismuth is generally considered safe when used as directed. Unlike many heavy metals, bismuth has very low toxicity. It’s commonly used in over-the-counter medicines for digestive issues. However, long-term use (over six weeks) should be avoided without medical supervision.
How does bismuth contribute to modern technology?
Bismuth plays a crucial role in modern technology, particularly in electronics and semiconductors. It’s used in thermoelectric materials for energy harvesting, in specialized chips for cooling devices, and is emerging as a promising material for 2D transistors, potentially leading to faster and more energy-efficient electronic devices.
Why is bismuth considered an environmentally friendly alternative to lead?
Bismuth is seen as a “green” alternative to lead because it has similar properties but much lower toxicity. It’s being used to replace lead in various applications such as fishing weights, ammunition, and electronics soldering. This shift helps reduce environmental contamination and health risks associated with lead use.
What makes bismuth crystals unique and visually appealing?
Bismuth crystals are known for their distinctive spiral staircase pattern and iridescent rainbow colors. These characteristics result from bismuth’s unique crystal growth process and the thin oxide layer that forms on the surface. The colorful, geometric formations make bismuth crystals popular among collectors and for use in decorative items.









